Carbon compounds which only contain the elements Hydrogren (H) and Carbon (C) are called hydrocarbons, all of which are organic (life-related) compounds. They are often found naturally in crude oil.
Engine Uses
Hydrocarbons are used as lubricant (known as motor oil) in an engine, as well as combustable material (in the form of both gasoline and diesel). Sometimes it is found in gaskets and other sealers.
 This involves chemical names, terms, and/or reactions, which may be complicated without a basic understanding of chemistry.
This involves chemical names, terms, and/or reactions, which may be complicated without a basic understanding of chemistry.Hydrocarbon Chemical Formulas
Hydrocarbons are a class of chemical, so there isn't one single chemical formula for them, unless you allow variables. The formula with variables is CnH2n+2. The chart below shows the name of the hydrocarbon assuming a single bond layout, or their "alkane" name. Different arrangements may have the center carbon atoms branch off instead of forming a straight line, and these branches are different chemicals with different properties. The "State" category notes the state of matter it is in at approximately 60°F at sea level atmospheric pressure.
| Alkane Name | Carbon | Hydrogen | Formula | State |
|---|---|---|---|---|
| Methane | 1 | 4 | CH4 | Gas |
| Ethane | 2 | 6 | C2H6 | Gas |
| Propane | 3 | 8 | C3H8 | Gas |
| Butane | 4 | 10 | C4H10 | Gas |
| Pentane | 5 | 12 | C5H12 | Liquid |
| Hexane | 6 | 14 | C6H14 | Liquid |
| Heptane | 7 | 16 | C7H16 | Liquid |
| Octane | 8 | 18 | C8H18 | Liquid |
| Nonane | 9 | 20 | C9H20 | Liquid |
| Decane | 10 | 22 | C10H22 | Liquid |
Octane
Octane is well-known for its relation to gasoline quality, but it is also a specific chemical, which is written as C8H18.
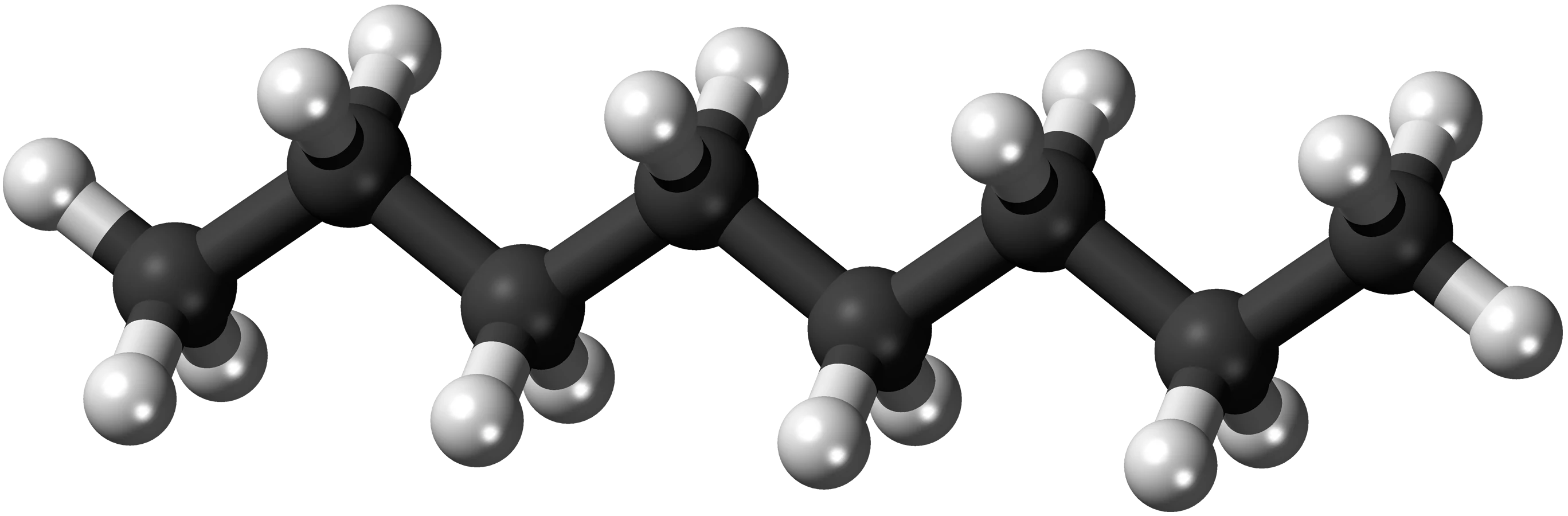
This image represents the chemical arrangement of Octane, an alkane hydrocarbon.
